GD2-CAR T Induces Tumor Regression in H3K27M-Mutant Diffuse Midline Gliomas
Phase 1 trial findings have revealed that GD2-CAR T-cell therapy can yield significant tumor regressions and neurological improvements in patients with H3K27M-mutant diffuse midline gliomas. A phase 1 clinical trial (NCT05316155) focused on investigating the use of GD2-targeted chimeric antigen receptor (CAR) T cells for treating H3K27M-mutant diffuse midline gliomas (DMGs) showed promising outcomes, indicating the feasibility, safety, and early efficacy of this therapeutic approach.
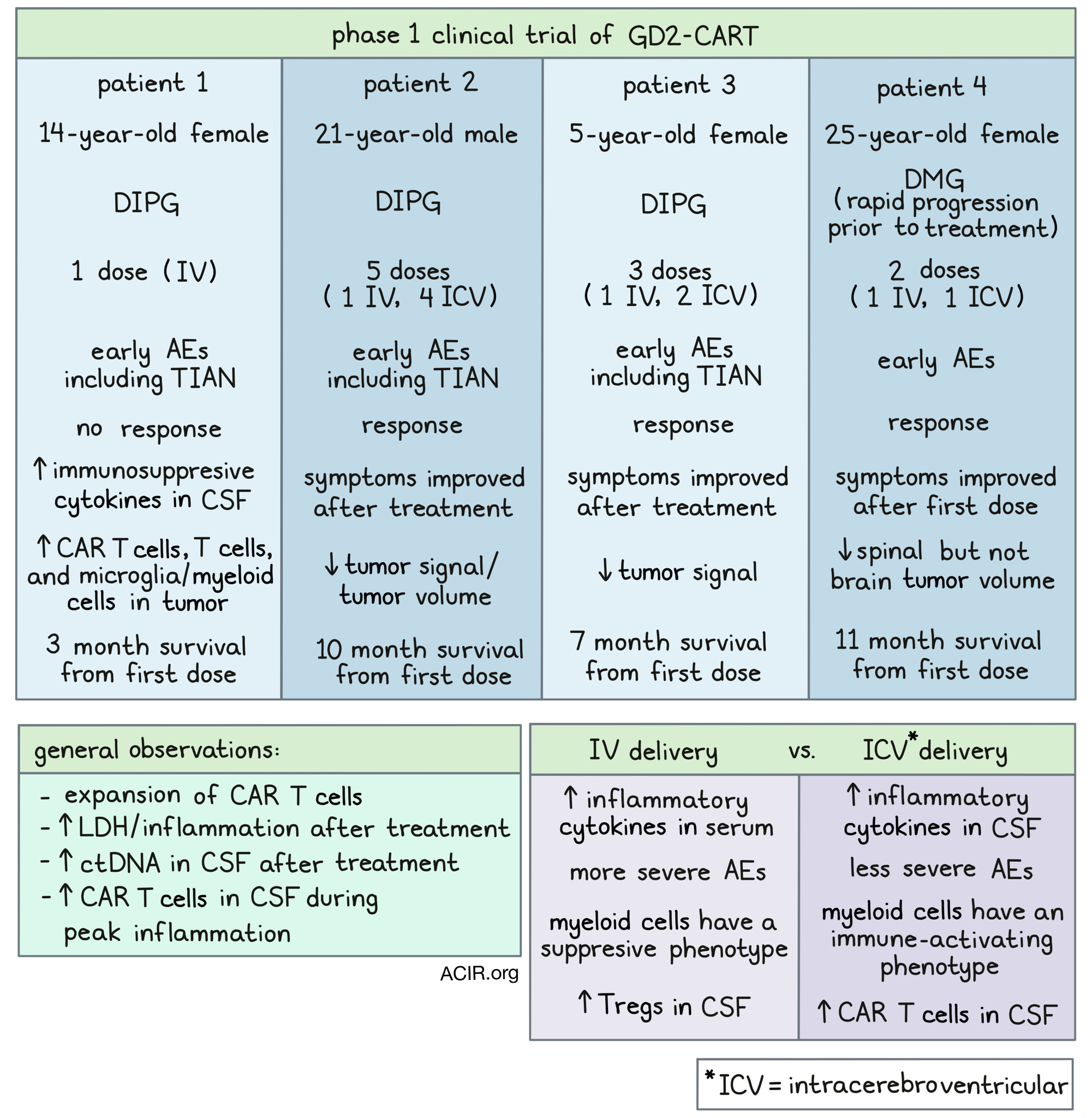
The trial included 13 patients, with 11 of them receiving GD2-CAR T-cell therapy. The results demonstrated substantial tumor regressions in several patients, with 4 individuals experiencing major reductions in tumor volume ranging from 52% to 100%. Notably, one patient achieved a complete response that persisted for over 30 months, while 9 other patients exhibited neurological improvement.
Manufacturing Feasibility and Safety
GD2-CAR T cells were effectively manufactured from immune cells derived from all enrolled subjects. Regarding safety, no dose-limiting toxicities (DLTs) were observed in the lower dose cohort of 1×106 cells/kg. However, in the higher dose cohort of 3×106 cells/kg, 3 patients experienced dose-limiting cytokine release syndrome, establishing the lower dose as the maximally tolerated intravenous (IV) dose.
Additional Treatments and Management
After the IV infusion, 9 patients received intracerebroventricular infusions of GD2-CAR T, which did not result in any additional DLTs. Nevertheless, all patients exhibited tumor inflammation-associated neurotoxicity, which was effectively managed through intensive monitoring.
"The present study establishes GD2-CAR T-cell therapy as a promising modality for a historically lethal central nervous system cancer. The rate of clinical improvements and tumor regressions, including sustained complete responses, warrant cautious optimism for CAR T-cell therapy of solid tumors," wrote the study authors in their findings published in Nature.
Study Objectives and Patient Criteria
The phase 1 study aimed to determine the successful generation of GD2-CAR T cells from immune cells obtained from pediatric and young adult patients with H3K27M-mutant diffuse intrinsic pontine glioma (DIPG) and spinal DMG. Eligible patients were required to undergo lymphodepleting chemotherapy before receiving a single IV dose of autologous GD2-CAR T cells at 2 dose levels.

Enrollment was open to patients aged 2 to 50 years with H3K27M-mutant DIPG or spinal DMG who had completed standard radiation therapy and chemotherapy. Patients needed to have normal organ and marrow function, along with specific performance score criteria.
Conclusion and Future Directions
A total of 13 patients participated in the trial, with 11 receiving treatment. The study's primary endpoints were manufacturing feasibility, safety, and identification of the maximally tolerated dose, while secondary endpoints included preliminary efficacy focusing on tumor response and neurological benefit.
Further clinical trials with larger patient cohorts are essential to validate the findings of this phase 1 study. Ongoing and future research will aim to optimize GD2-CAR T-cell therapy for more complete and durable responses in individuals with H3K27M-mutated diffuse midline gliomas, both in pediatric and adult populations.










