Switch Maintenance Paclitaxel/Ramucirumab Prolongs Survival in HER2-Negative Metastatic Gastric Cancer
Switch maintenance therapy with paclitaxel (Taxol) plus ramucirumab (Cyramza) has shown consistent prolongation of survival in HER2-negative disease controlled, metastatic gastric cancer. The phase 3 ARMANI trial (NCT02934464) revealed significant improvements in progression-free survival (PFS) and overall survival (OS) with paclitaxel and ramucirumab combination therapy, as presented at the European Society for Medical Oncology Gastrointestinal (ESMO GI) Cancer Congress 2024.
Patients with HER2-negative advanced gastric or gastroesophageal junction (GEJ) cancer, who had no disease progression after 3 months of initial oxaliplatin-based chemotherapy, were enrolled in the trial. One group received ramucirumab plus paclitaxel (arm A), while the other group received additional chemotherapy followed by maintenance therapy with fluoropyrimidine (arm B).
Treatment and Patient Characteristics
In arm A, patients received paclitaxel and ramucirumab as per the specified dosages and schedule. The majority of patients in both arms were male, with similar ECOG performance statuses and site of origin distribution. Metastatic sites and prior gastrectomy rates were also comparable between the two treatment arms.
Biomarker Evaluations
The trial included an exploratory analysis of biomarkers such as PD-L1 combined positive score (CPS), Claudin 18.2 (CLDN18), and mismatch repair (MMR) status to predict treatment response and outcomes. Various subgroups based on biomarker status showed differing median PFS and OS results, emphasizing the impact of biomarkers on treatment efficacy.
The Role of Angiogenesis Targeted Therapies in Gastric Cancer
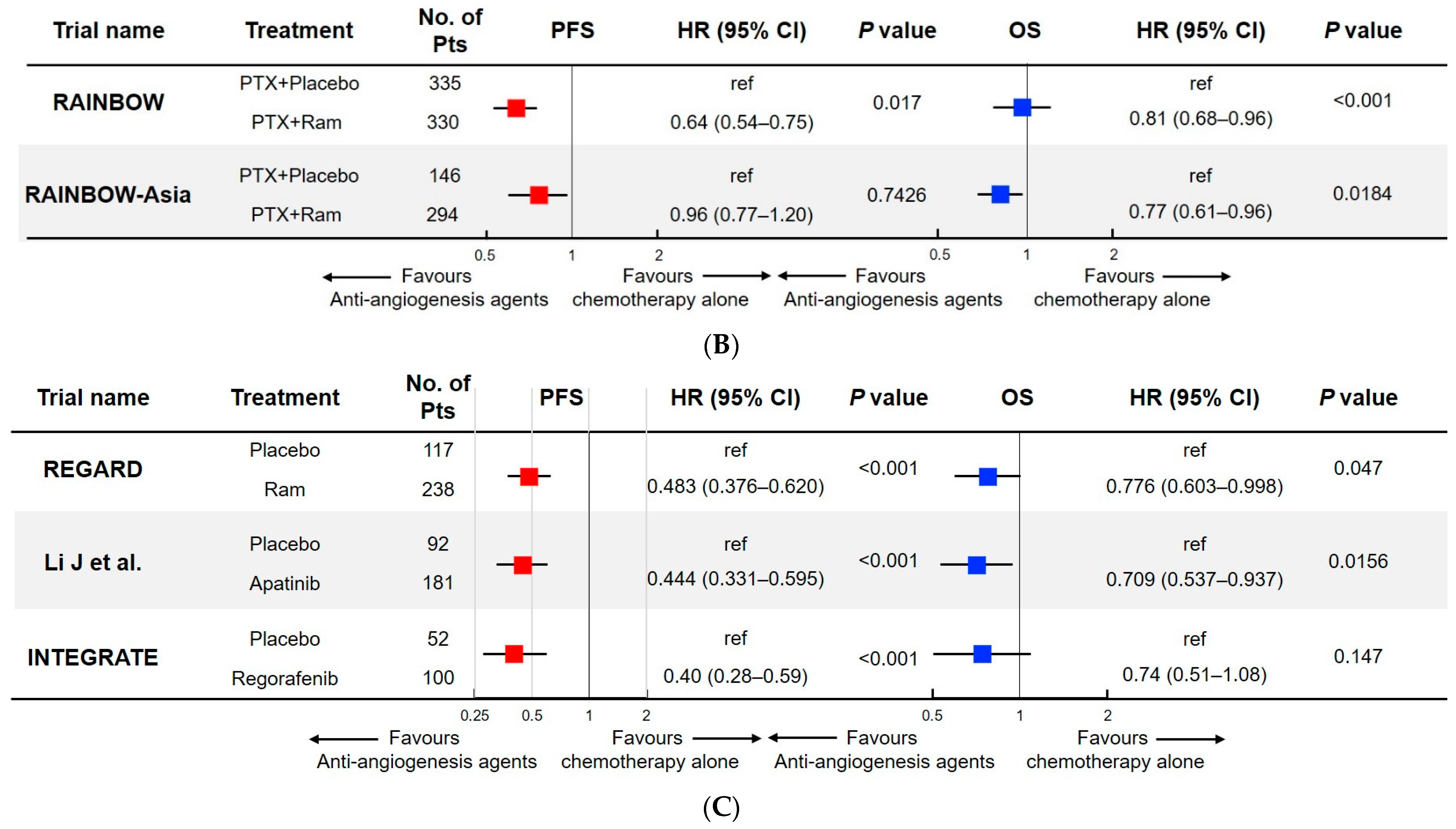
Significance of Findings
The study highlighted the survival benefits of paclitaxel plus ramucirumab in patients with HER2-negative metastatic gastric cancer. The interaction between biomarker status and treatment efficacy further underscored the importance of personalized medicine in oncology.
Overall, the ARMANI trial represents a promising post-induction therapy option for patients ineligible for targeted agents, with implications for first-line treatment decisions and biomarker testing.

Contact Us: 2 Commerce Drive, Cranbury, NJ 08512 | 609-716-7777




















