Biosimilar Drugs Could Generate $38.4 Billion in Savings over Five Years
Biosimilar drugs have the potential to significantly reduce prices for expensive medications used in the treatment of conditions such as cancer and rheumatoid arthritis. A recent study conducted by the RAND Corporation projects savings of $38.4 billion, equivalent to 5.9% of the total projected spending on biologics in the United States from 2021 to 2025.
Potential for Larger Savings
The study suggests that more widespread adoption and competition among biosimilar drugs could lead to even greater cost reductions. Under the most optimistic scenario, savings could reach as high as $124.5 billion over the same five-year period.
The anticipated savings are primarily attributed to the competitive pressure biosimilars exert on brand-name biologics, rather than lower pricing of the biosimilar drugs themselves. 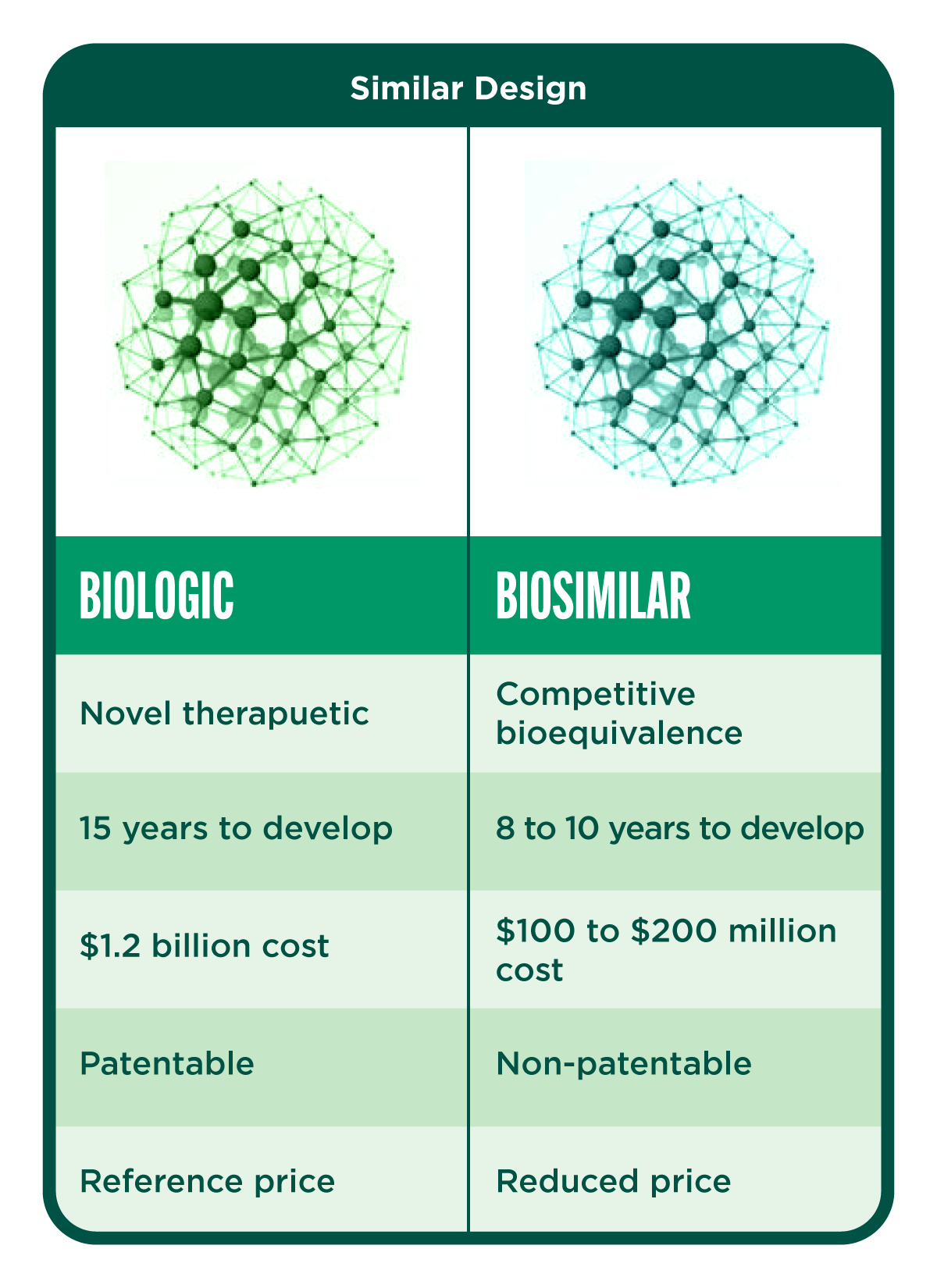
According to lead author Andrew W. Mulcahy of the RAND Corporation, "Biosimilars have the potential to lower spending on biologic drugs that account for a rapidly increasing share of overall US prescription drug spending."
Understanding Biologics and Biosimilars
Biologics are complex drugs produced in living organisms and are used in the treatment of various diseases, including cancer, multiple sclerosis, and rheumatoid arthritis. Despite accounting for only 2% of prescriptions by volume in 2017, biologics represented 37% of total prescription drug spending in the US.
Biosimilars are highly similar to approved reference biologics in terms of efficacy, safety, and potency but are manufactured by different companies. They receive approval from the FDA after the exclusivity period of the reference biologic expires.
Implications for Healthcare Policy
The study's findings suggest that policies promoting the increased use of biosimilars could yield substantial savings for the US healthcare system. Potential policy changes include equal reimbursement rates for reference biologics and biosimilars, higher margins for biosimilars, and reduced out-of-pocket costs for patients using biosimilars.
Policy proposals aimed at enhancing biosimilar uptake are currently being considered by lawmakers and policymakers at the federal level.
Further research is needed to assess the impact of specific policy interventions and to understand how factors such as market competition and regulatory frameworks influence the potential savings from biosimilars. 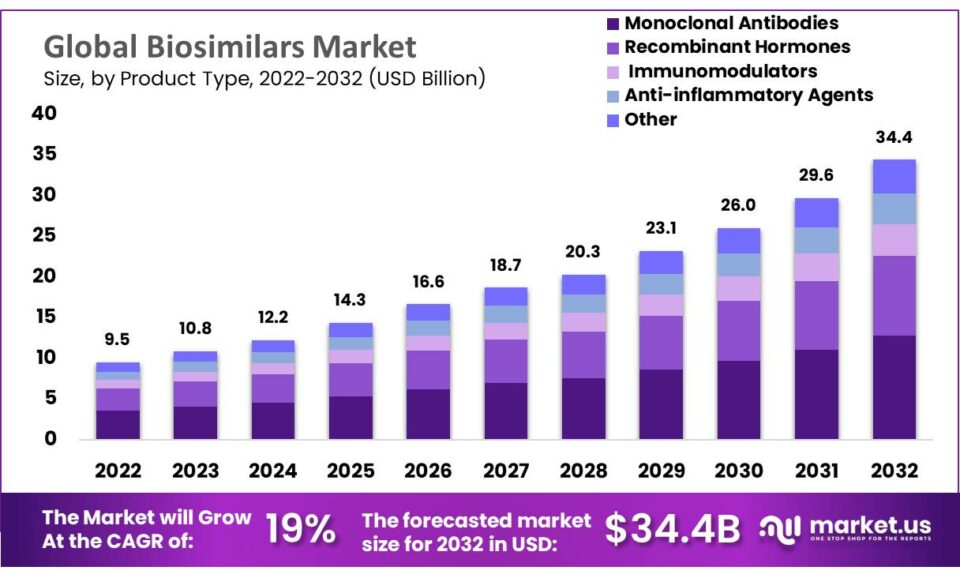
Support for the study was provided by the Office of the Assistant Secretary for Planning and Evaluation within the US Department of Health and Human Services.
For more information on the study, you can read the full publication in the American Journal of Managed Care.




















