Zenocutuzumab Now FDA-Approved in NRG1+ NSCLC and ...
With the recent FDA approval, zenocutuzumab has emerged as the first FDA-approved therapy specifically designed for patients with NRG1 fusion tumors. This groundbreaking approval marks a significant milestone in the treatment landscape for these types of cancers.
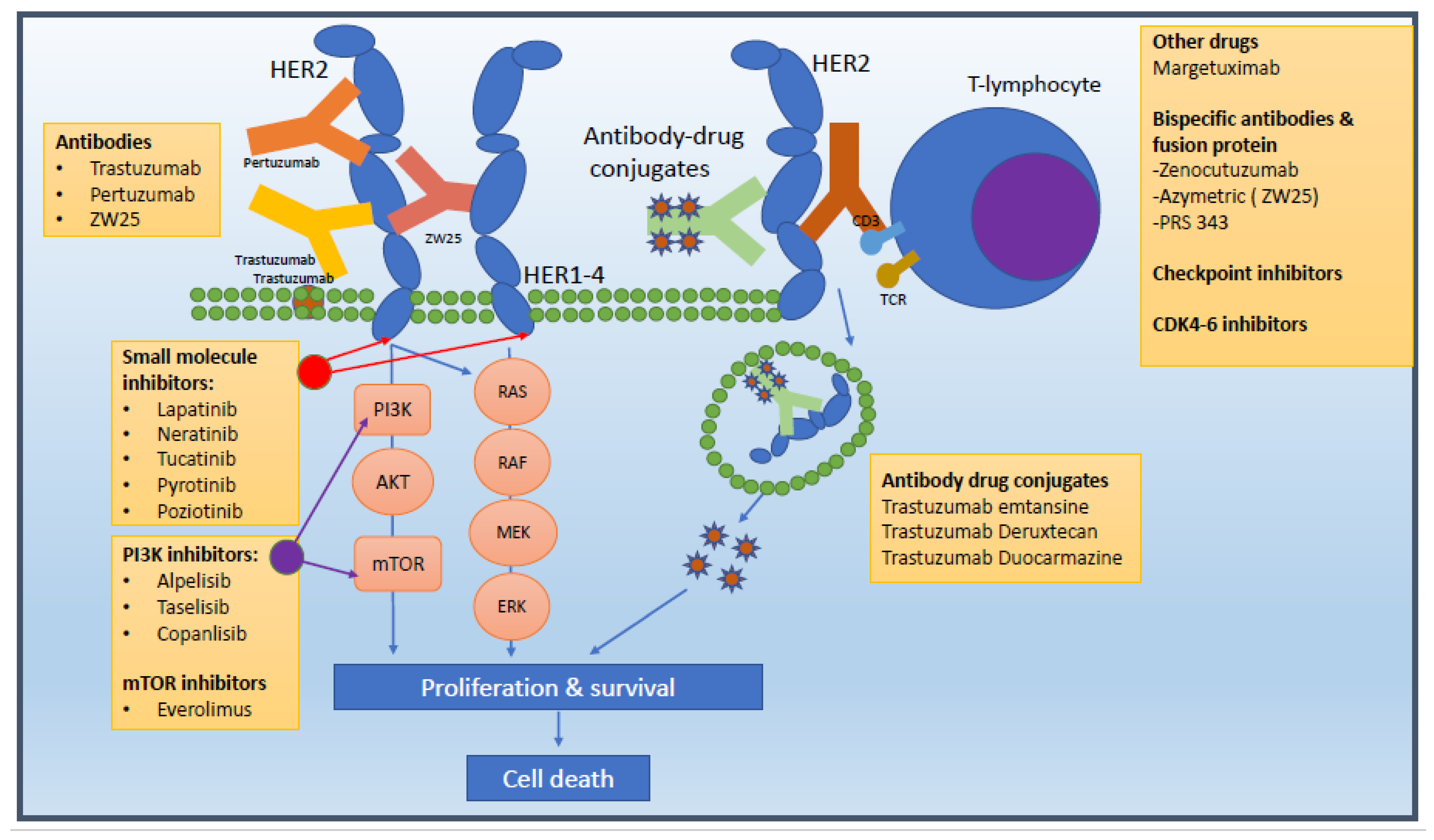
Approval Process and Designation
The FDA's approval of zenocutuzumab signifies a crucial advancement in targeted therapy for patients with NRG1+ NSCLC and PDAC. The biologics license application of zenocutuzumab underwent a priority review in May 2024, emphasizing the urgency and importance of this treatment option. Additionally, the agent was granted breakthrough therapy designation in July 2023, further highlighting its potential impact in the field of oncology.
Phase 1/2 eNRGy Trial Results
The approval of zenocutuzumab was supported by data from the phase 1/2 eNRGy trial, which assessed the safety and efficacy of this innovative therapy. The trial focused on key outcome measures such as the overall response rate (ORR) and duration of response (DOR), providing valuable insights into the treatment's effectiveness.
For patients with NSCLC, the trial demonstrated an ORR of 33% and a median DOR of 7.4 months. Similarly, for individuals with pancreatic adenocarcinoma, the ORR was 40% with a DOR ranging from 3.7 to 16.6 months. These findings underscore the potential of zenocutuzumab in addressing the needs of patients with NRG1 fusion tumors.
Safety Profile and Adverse Events
As with any therapeutic intervention, the safety profile of zenocutuzumab is of paramount importance. Common adverse events observed during the trial included diarrhea, musculoskeletal pain, fatigue, and infusion-related reactions.
Additionally, specific laboratory abnormalities were noted, highlighting the need for close monitoring and management of potential side effects.
Scientific Presentations and Clinical Data
Data from the eNRGy trial were presented at prominent oncology conferences, including the American Society of Clinical Oncology (ASCO) Annual Meeting and the European Society for Medical Oncology (ESMO) Congress. These presentations showcased the significant impact of zenocutuzumab in patients with NRG1+ cancers, further validating its efficacy and safety profile.
Future Implications and Clinical Trials
The success of zenocutuzumab in NRG1+ NSCLC and PDAC opens up new possibilities for patients with these challenging conditions. Ongoing clinical trials, such as the eNRGy study, continue to explore the full potential of this therapy in diverse patient populations, offering hope for improved outcomes and quality of life.




















